Transforming common insulators into superior superconductors
A Detailed comparative study on the structures and electrical properties of related insulators and superconductors
The organic compound in this study is puzzling because it controversially exhibits properties of both superconductors (zero resistivity) and insulators (infinite resistivity). Based on detailed comparison of the structures and electrical properties of a series of single crystals of the same material, we have discovered that the differences between superconductors and insulators are governed by the slightest difference in a part of the molecular structure, which is not directly included in the electrical conduction pathways.
Superconductors are a limited group of materials which conduct electricity with zero resistance at lower than certain temperatures (TCs) peculiar to each material. Once zero resistance is achieved, the loss of electric energy becomes zero during the transmission and consumption of electricity. The application of these superconductors may enable future technology, such as an ultra-high speed transportation system. However, the TCs for known superconductors are too low (~ -250 °C, for example) to be applied. The requirement for superconductors, i.e., what makes common substances transform into high-TC superconductors, is the key to their application in future technologies. This report is a case study focusing on revealing that key factor.
The material is a superconductor with a rather high TC for organic compounds, but for the past thirty years it has been controversially reported to be both a superconductor and a non-superconductor. If we reveal the origin of the inconsistency and the differences between the samples with the opposite properties, we will know the key factor in becoming a (high-TC) superconductor. After we examined the detailed structures and electrical properties of a series of different samples of this material, we discovered one important fact; even the part of the molecules not involved in the conduction pathways could qualitatively have an effect on the (super)conducting properties. Only the samples containing the required molecular structures exhibited zero-resistance at low temperatures. This finding indicates that one should carefully design the molecular structures when developing high-TC superconductors, whether they are involved in conduction pathways or not.
Reference URL: https://doi.org/10.1039/D1MA00933H
Bibliographic Information
Organic charge transfer complex at the boundary between superconductors and insulators: critical role of a marginal part of conduction pathways. Toshio Naito, Hayato Takeda, Yusuke Matsuzawa, Megumi Kurihara, Akio Yamada, Yusuke Nakamura and Takashi Yamamoto. Materials Advances 3, 1506-1511(2022). DOI: 10.1039/D1MA00933H. Advanced Online Publication on 2021 (December 15), Published on 2022 (February 8).
Fundings
- Grant-in-Aid for Challenging Exploratory Research (18K19061) and Grant-in-Aid for Scientific Research (C) (15K05478, 19K05405) of JSPS
- Tokyo Chemical Industry Foundation
- Tokyo Ohka Foundation for the Promotion of Science and Technology
- Kato Foundation for Promotion of Science
- Iketani Science and Technology Foundation (ISTF; 0331005-A)
- Casio Science Promotion Foundation
- Ehime University Grant for Project for the Promotion of Industry/University Cooperation
Media
-
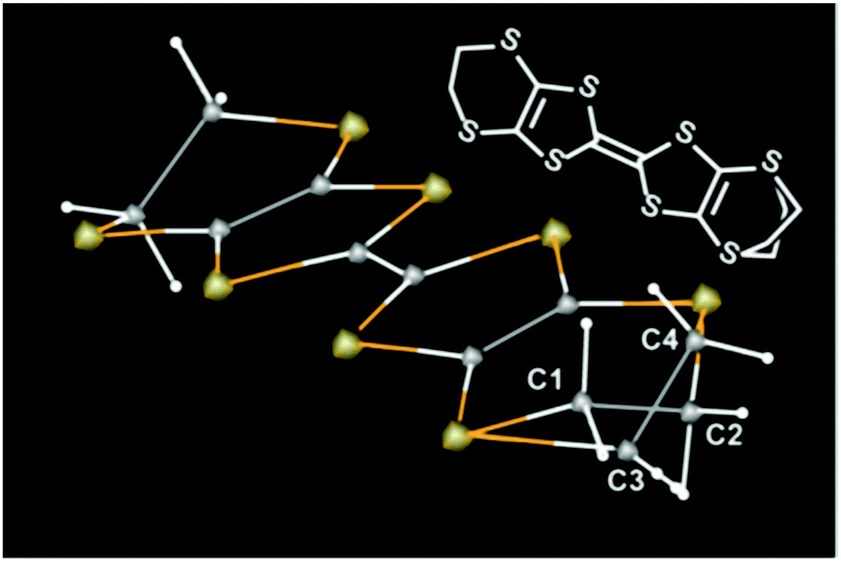
The molecular structures dominating the superconductivity revealed in this study
The gold, grey, and white cubes or spheres designate sulfur (S), carbon (C), and hydrogen atoms, respectively. The electric current flows mainly through the sulfur atoms. The molecular structure is schematically shown in the top right in the figure. The complicated arrangement of atoms around C1, C2, C3, and C4 disturbs the electric current, which, at a low temperature, governs whether the material becomes superconducting or not. (This figure is reproduced from Fig. 3 in the original paper of this article)
credit : Toshio Naito, Ehime University
Usage Restriction : Please get copyright permission -
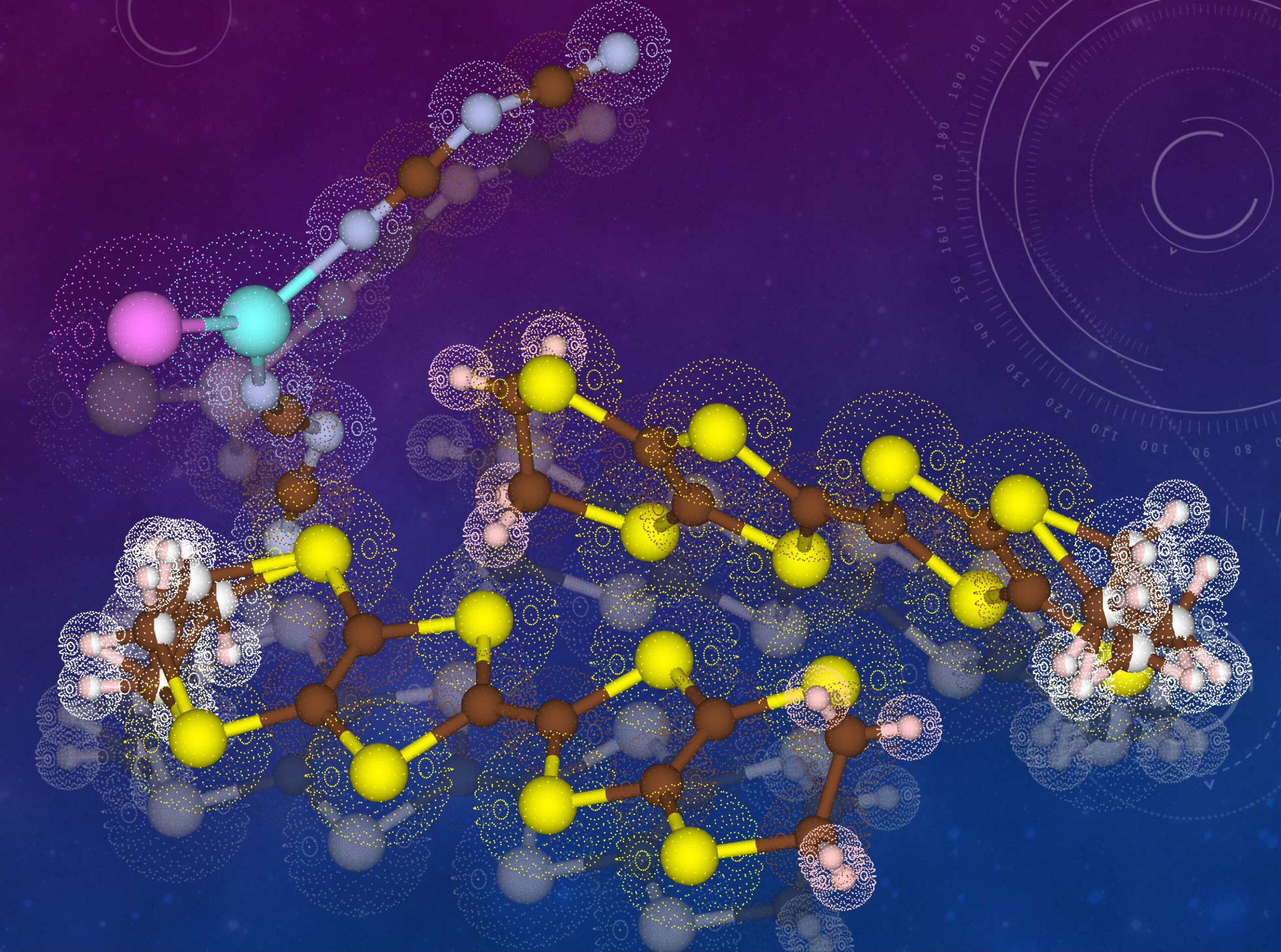
The molecular structures and electron densities revealed in this study (1)
The molecular structures and electron distributions in an intriguing superconductor exhibits properties of both superconductors and non-superconductors, depending on the synthetic and measurement conditions. This material happens to be located between superconductors and non-superconductors and is the sample of choice for investigation of the requirements for superconductors. The electric current flows from top right to bottom left mainly through the sulfur atoms, shown by the yellow spheres. The complicated arrangement of atoms shown by the pale pink and white spheres seriously affects the electric current and superconductivity of this material.
credit : Toshio Naito, Ehime University
Usage Restriction : Please get copyright permission -
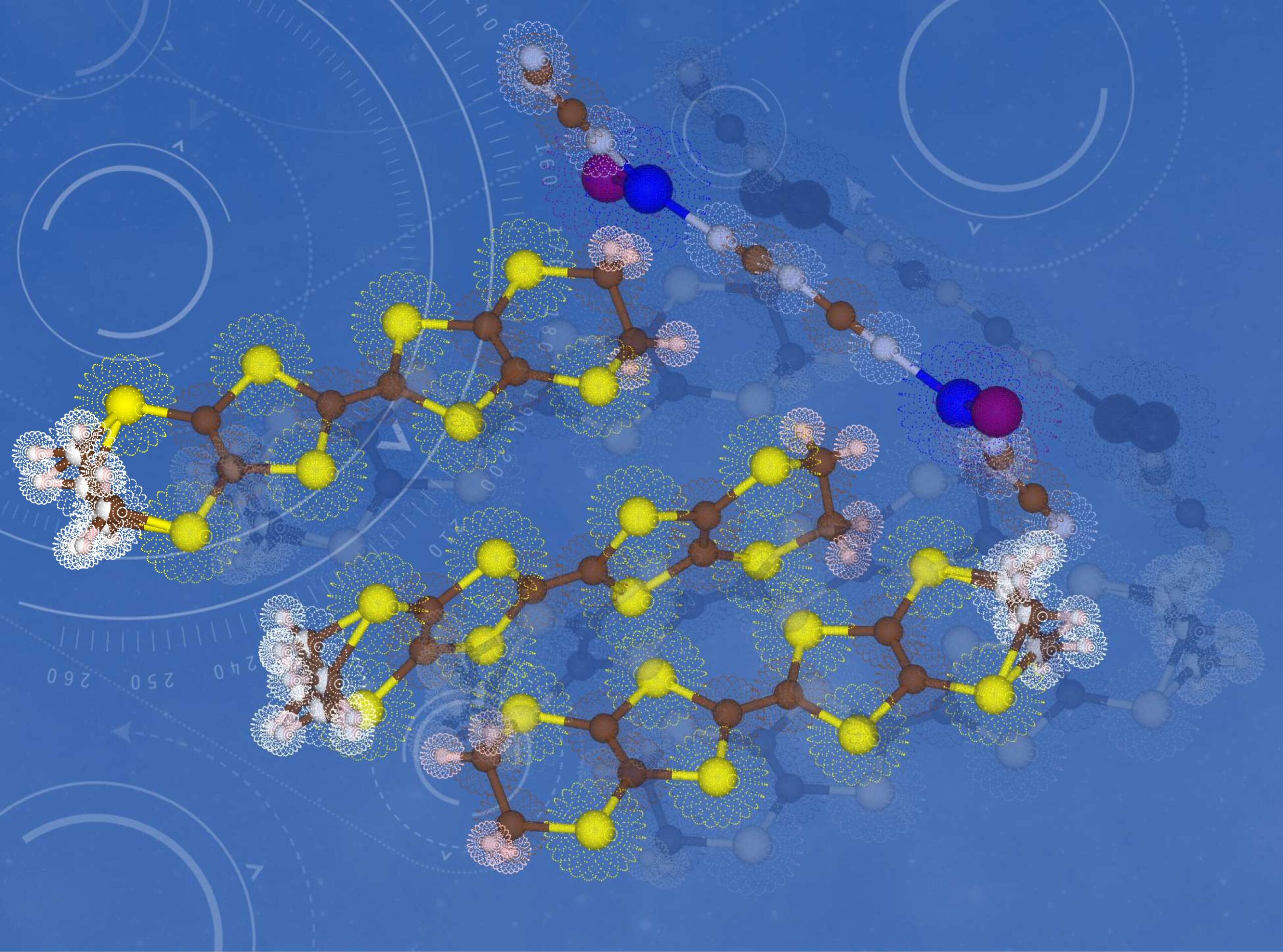
The molecular structures and electron densities revealed in this study (2)
The molecular structures and electron distributions in an intriguing superconductor exhibits properties of both superconductors and non-superconductors depending on the synthetic and measurement conditions. This material happens to be located between superconductors and non-superconductors and is the sample of choice for investigation of the requirements for superconductors. The electric current flows from top left to bottom right mainly through the sulfur atoms shown by the yellow spheres. The complicated arrangement of atoms shown by pale pink and white spheres seriously affects the electric current and superconductivity of this material.
credit : Toshio Naito, Ehime University
Usage Restriction : Please get copyright permission
Contact Person
Name : Toshio Naito
Phone : +81-89-927-9604
E-mail : tnaito@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering
