Synthesis and Properties of Wing-Shaped Nanographene
Aromaticity of the Space Surrounded by Two Aromatic Rings
・Synthesization of spatially arranged Wing-Shaped Nanographene (HPHAC dimers) based on a Rigid Bicyclooctadiene framework.
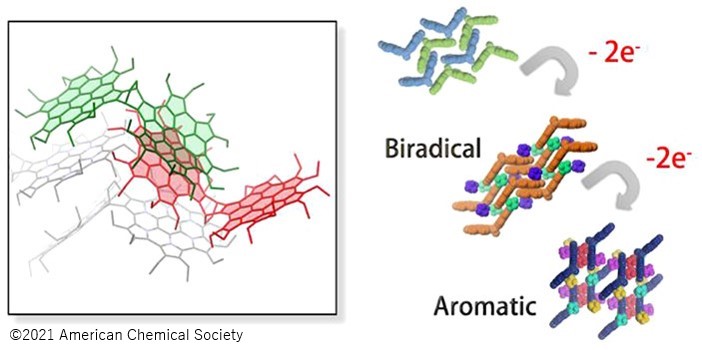
・Each HPHAC unit showed multi-step redox properties, and the assembly modes of the dimer were revealed to be dependent on its oxidation state.
・Increased aromaticity was suggested in the space surrounded by the two HPHAC.
In recent years, various synthetic chemical approaches have been investigated using polycyclic aromatic compounds (PAHs) as “nanographene with precise molecular structure and high purity”. If it becomes possible to construct structurally controlled 3D assemblies and spaces from this 2D nano-sized graphene, new materials with unique structural properties can be created. A research group at Ehime University has been studying the synthesis and physical properties of hexapyrrolohexaazacoronene (HPHAC), a nitrogen-containing PAH, using pyrroles. HPHACs, which are composed of electron-rich pyrroles, are easily oxidized and their two-electron oxidized forms exhibit global aromaticity. However, the only reported studies on HPHACs have been on the synthesis of monomers, including analogues, and the elucidation of their structure-property relationships. No studies have been conducted on the synthesis of dimers toward structurally controlled 3D structures.
In this study, a wing-shaped HPHAC dimer was synthesized by employing a bicyclooctadiene skeleton as a structurally rigid crosslinking site. As with the known HPHAC monomer, stable redox properties were observed in the newly synthesized dimer. In addition, the characteristic assembly modes depending on the oxidation state were revealed by single crystal structure analysis and various spectroscopic measurements. Furthermore, in the tetracationic species of the HPHAC dimer, the aromaticity of the 3D space surrounded by the two HPHACs was found to be enhanced. This is a result of the influence of the magnetic and electronic properties of the HPHACs on each other.
In recent synthetic chemical research on nanographene, instead of the conventional planar compounds, high-dimensional compounds with, for example, bowl- and saddle-shaped structures have been reported. On the other hand, there have been few studies on the construction of structurally controlled 3D assemblies and spaces and their use as constituent units. The versatile assembly modes of 3D nanographene can provide high mechanical robustness and a large surface area, and thus are expected to be applied to sensors, bioimaging, and energy conversion materials.
Reference URL: https://pubs.acs.org/doi/10.1021/acs.orglett.1c03669
Bibliographic Information
Synthesis, Properties, and Packing Structures of Wing-Shaped N-Doped Nanographene in Various Oxidation States, Fan Wu, Kosuke Oki, Jiaying Xue, Shigeki Mori, Masayoshi Takase, Zhen Shen and Hidemitsu Uno, Organic Letters, 2022, 24, 80-84, doi:10.1021/acs.orglett.1c03669 (December 13).
Fundings
- JSPS KAKENHI JP20H02725, JP19K05422
- NSFC-JSPS joint project JPJSBP120197420, 21911540069
- National Natural Science Foundation of China 22001119, 21771102, 22071103
Media
Contact Person
Name : Masayoshi Takase, Hidemitsu Uno
Phone : +81-89-927-9612
E-mail : takase.masayoshi.ry@ehime-u.ac.jp, uno@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering, Ehime University