Synthesis of non- or antiaromatic conjugated macrocycles
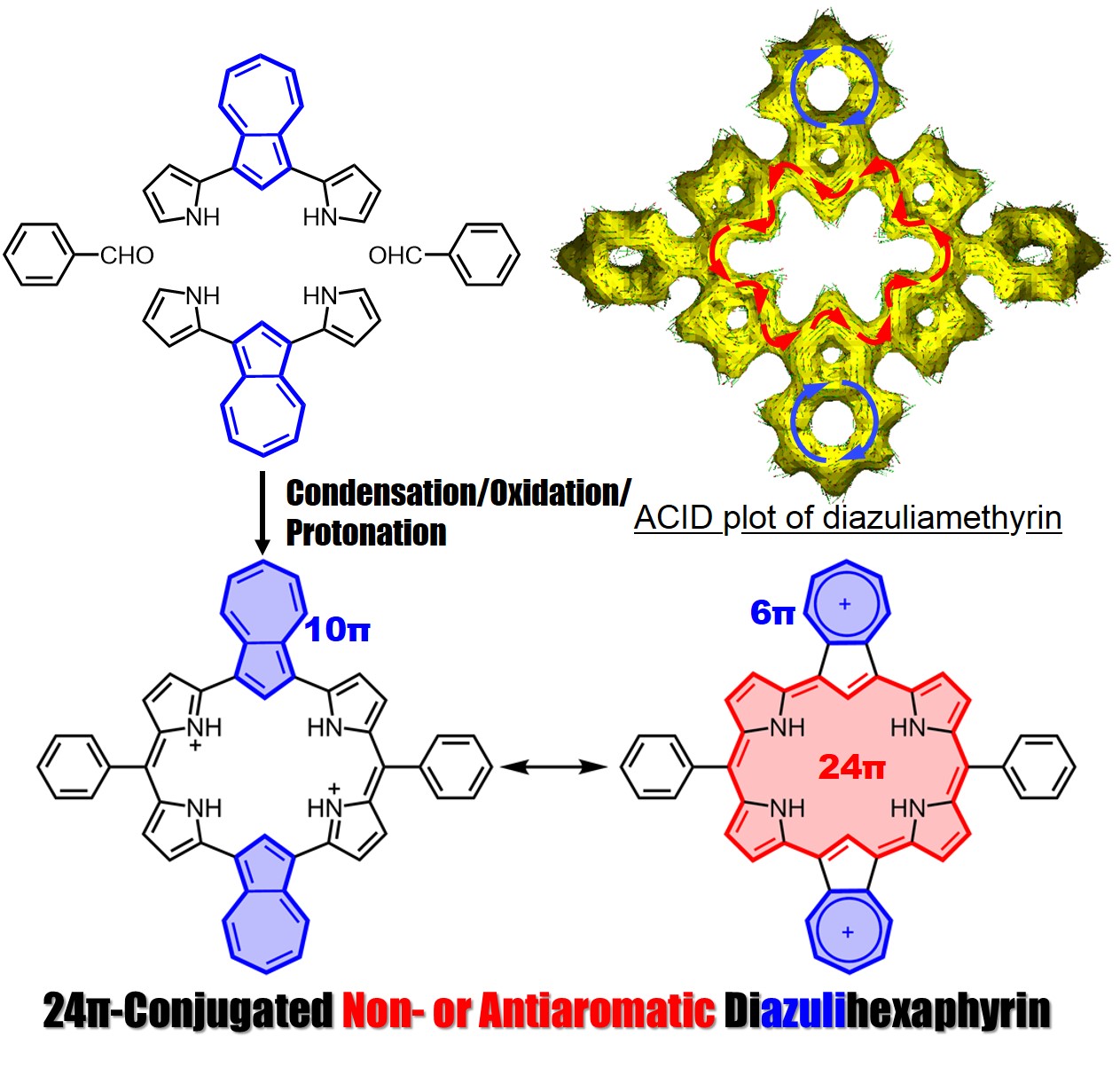
Azulene-containing ring-expanded porphyrin with a 24pi-system
A ring-expanded porphyrin, diazuliamethyrin, was successfully synthesized via a “3+3” condensation method. This porphyrin showed a 24pi non- or antiaromatic character. We analyzed the optical and electronic structures using magnetic circular dichroism spectroscopy and time-dependent density functional theory calculations.
Profs. Okujima and Uno, in collaboration with Prof. Kobayashi at Shinshu University, reported their success in the synthesis of diazuliamethyrin, a core-modified hexaphyrin(1.0.0.1.0.0), and descibed its molecular structure, electronic structure and optical properties.
Amethyrin is a stable and antiaromatic ring-expanded porphyrin comprised of 6 pyrroles and 2 meso-bridged carbons. We successfully synthesized diazuliamethyrin via a “3+3” porphyrin synthesis. We confirmed it to have a 24pi non- or antiaromatic character by NMR, absorption, and MCD spectra analyses, and by TD-DFT calculations. Our findings were published on December 21, 2021 in Organic Letters.
Reference URL: https://pubs.acs.org/doi/10.1021/acs.orglett.1c03882
Bibliographic Information
Journal: Organic Letters
Title: Synthesis of Non- or Antiaromatic Dicarbaamethyrin: [24]Diazulihexaphyrin(0.1.0.0.1.0)
Authors: Tetsuo Okujima, Hayato Inaba, Shigeki Mori, Masayoshi Takase, Hidemitsu Uno, Yoshiaki Chino, Yusuke Okada, Nagao Kobayashi
DOI: 10.1021/acs.orglett.1c03882
Media
Contact Person
Name : Tetsuo Okujima, Hidemitsu Uno
Phone : +81-89-927-9615
E-mail : okujima.tetsuo.mu@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering