Double Aromatic Rings Stabilize Multications
A nitrogen-embedded polycyclic aromatic hydrocarbon which shows reversible redox properties
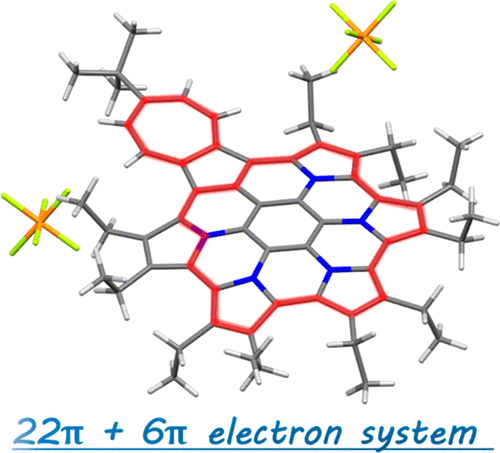
Polycyclic heteroaromatic molecules are a highly useful class of organic materials. In this study, synthesis of a new azacoronene, in which both pyrrole and azulene moieties are circularly fused, was achieved in just three steps. This new azacoronene exhibited multistep reversible oxidations under electrochemical and chemical conditions. Formation of an aromatic 22π-electron conjugation and a tropylium cation (6π-electron conjugation) in the dicationic state played a key role in stabilizing this cationic species.
A redox active polycyclic aromatic hydrocarbon (PAHs) composed of azulene and pyrroles was developed by a research team at Ehime University. This nitrogen-embedded PAH contains two kinds of aromatic rings in the dicationic state which can stabilize cations by delocalization based on global aromaticity. The findings were published on March 5, 2019 in Organic Letters.
Preparation of novel PAHs with well-defined structures has gained enormous interest in the creation of next-generation materials. Redox active PAHs are especially important for application such as organic electronics. Based on this background, the team had reported pyrrole-fused azacoronene, i.e., hexapyrrolohexaazacoronene (HPHAC), where six pyrrole rings were fused to a coronene core. Due to the circularly connected pyrrole rings, oxidized species were reversibly obtained, exhibiting global aromaticity in the dicationic state by forming a 22π-electron conjugation. In this study, pyrrole- and azulene-fused azacoronene was newly designed and synthesized. The electron-deficient seven-membered ring of azulene was expected to stabilize a cationic charge by forming an aromatic tropylium cation. In fact, crystal structure analysis, as well as DFT calculations, revealed both an aromatic 22π-electron conjugation and a tropylium cation (6π-electron conjugation). The control of charge resonance is an important design strategy in creating organic functional materials . The present contribution opens up the study of PAH chemistry.
Reference URL: https://pubs.acs.org/doi/abs/10.1021/acs.orglett.9b00515
Bibliographic Information
Synthesis and Redox Properties of Pyrrole- and Azulene-Fused Azacoronene, Yoshiki Sasaki, Masayoshi Takase, Tetsuo Okujima, Shigeki Mori, Hidemitsu Uno, Organic Letters, 20019, 21, 1900-1903, doi:10.1021/acs.orglett.9b00515 (March 5, 2019).
Fundings
- Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP16K05698
Media
Contact Person
Name : Masayoshi Takase, Hidemitsu Uno
Phone : +81-89-927-9612
E-mail : takase.masayoshi.ry@ehime-u.ac.jp, uno@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering