Nitrogen-Embedded Polycyclic Compound with Strong Antiaromaticity and Stability
Expedited Approach toward Antiaromatic Compounds
Nitrogen-embedded polycyclic compounds with strong antiaromaticity and stability were synthesized and isolated for the first time using pyrrole as a key unit. An expedited approach toward stable antiaromatic polycyclic compounds enables not only the revealing of its fundamental properties, but also its application to organic electronic materials.
The synthesis and isolation of a nitrogen-embedded polycyclic compound with strong antiaromaticity and stability was achieved for the first time by a research group at Ehime University. This result was published on October 2, 2019 in the Journal of the American Chemical Society.
Aromaticity is one of the most important concepts in chemistry, which has the influence on fundamental properties of cyclic conjugated compounds. In general, compounds with 4n+2 π electrons in the ring are stable due to their aromatic nature, and are widely used around us: from plastics to pharmaceuticals, and from dyes to organic electronics materials. On the other hand, antiaromatic compounds with 4n π electrons in the ring lack stability, and thus research on the synthesis and characterization of such compounds remains to be elucidated. In this report, an expedited approach toward a nitrogen-embedded antiaromatic compound with strong antiaromaticity and stability is presented. The new compounds were efficiently synthesized in just three steps from commercially available reagents via a substitution reaction and successive intramolecular Schooll and Vilsmeier-type reactions. Detailed investigation revealed its strong antiaromaticity and stability even in air.
Recently, polycyclic compounds have been studied as graphene-like molecules with a discrete structure. But compounds with aromaticity and antiaromaticity have been limited mainly to annulenes and porphyrins. This report presents the first nitrogen-embedded polycyclic compound with antiaromaticity. The easy access to such compounds enables not only the revealing of its fundamental properties but also its application to organic electronic materials.
Reference URL: https://pubs.acs.org/doi/10.1021/jacs.9b09260
Bibliographic Information
Synthesis and Isolation of Antiaromatic Expanded Azacoronene via Intramolecular Vilsmeier-Type Reaction, Kosuke Oki, Masayoshi Takase, Shigeki Mori, Hidemitsu Uno, Journal of the American Chemical Society 2019, 141, doi:10.1021/jacs9b09260 (October 2, 2019).
Fundings
- Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP16K05698
Media
-
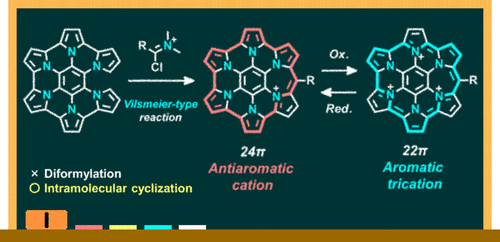
Synthesis of antiaromatic expanded azacoronene via intramolecular Vilsmeier-type reaction which shows aromaticity switching upon redox stimuli
credit : Figure Adapted with Permission from Journal of the American Chemical Society 2019, 141. © 2019 American Chemical Society.
Usage Restriction : Please get copyright permission
Contact Person
Name : Masayoshi Takase, Hidemitsu Uno
Phone : +81-89-927-9612
E-mail : takase.masayoshi.ry@ehime-u.ac.jp; uno@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering
