Molecules bend, properties change! Nanographene transforms through oxidation
Structural changes and electronic properties of pyrrole-fused aza-nanographene revealed based on oxidation state
The collaborative research group led by Ehime University have synthesized a novel pyrrole-fused aza-nanographene that bends into a ladder-shaped structure due to its unique “gulf-edge” regions. The molecule shows remarkable oxidation-dependent electronic behavior: a diradical open-shell dication and a globally aromatic closed-shell tetracation. This finding reveals how molecular curvature and oxidation state govern electronic properties, paving the way for designing redox-active π-conjugated materials and organic molecular switches.
Graphene and its molecular fragments, known as nanographenes, are key materials for next-generation organic electronics due to their tunable π-electron frameworks. The physical and electronic properties of nanographenes are highly sensitive to molecular size, shape, and especially edge topology. Among the various edge types, “gulf edges”—deep concave sites on the molecular boundary—have remained largely unexplored despite their potential to induce strong curvature and unique electronic behavior.
The collaborative research group led by Ehime University designed and synthesized a nitrogen-containing nanographene derivative, fused octapyrrolylanthracene(fOPA), by introducing eight pyrrole rings onto an anthracene skeleton. This new molecule was obtained in only two synthetic steps. X-ray crystallographic analysis revealed that steric repulsion between hydrogen atoms at the gulf-edge regions induces a natural bending of the molecule, leading to a ladder-like conformation. Quantum chemical calculations further confirmed that this nonplanar structure is more stable than the twisted alternative.
Electrochemical studies showed that fOPA undergoes up to four reversible oxidation processes. Upon stepwise oxidation, the molecule’s electronic character changes dramatically. The dicationic species(fOPA2+) adopts a singlet diradical configuration with two spatially separated spins localized at the gulf-edge regions, while the tetracationic species(fOPA4+) exhibits a closed-shell aromatic structure accompanied by global diatropic ring currents. These features were verified through ESR and NMR spectroscopy, along with ACID and NICS computational analyses.
The discovery of such oxidation-dependent switching between open-shell and closed-shell electronic states within a single curved π-framework demonstrates a new concept in molecular electronics—where structural flexibility and redox activity are inherently coupled. This could open pathways to novel organic conductors, molecular switches, and responsive optoelectronic materials.
Reference URL: https://pubs.acs.org/doi/10.1021/acs.orglett.5c03278
Bibliographic Information
Title:Pyrrole-Fused Aza-Nanographene with Two Gulf-Edge Regions as Flexible Sites
Authors:Takayuki Matsunaga(Ehime Univ.), Kosuke Oki(Ehime Univ.), Shigeki Mori(Ehime Univ.), Tomohiko Nishiuchi(Osaka Univ.), Masahiro Higashi(Nagoya Univ.), Takashi Kubo(Osaka Univ.), Tetsuo Okujima(Ehime Univ.), Hidemitsu Uno(Ehime Univ.), and Masayoshi Takase*(Ehime Univ.)(*corresponding author)
Journal:Organic Letters, 2025, 27, 11005–11010
DOI:10.1021/acs.orglett.5c03278
Publication Date:September 8, 2025
Fundings
- Japan Society for the Promotion of Science KAKENHI(JP24K01470, JP23H03964, JP20H02725, JP20H05839)
- Nagase Science and Technology Foundation
Media
-
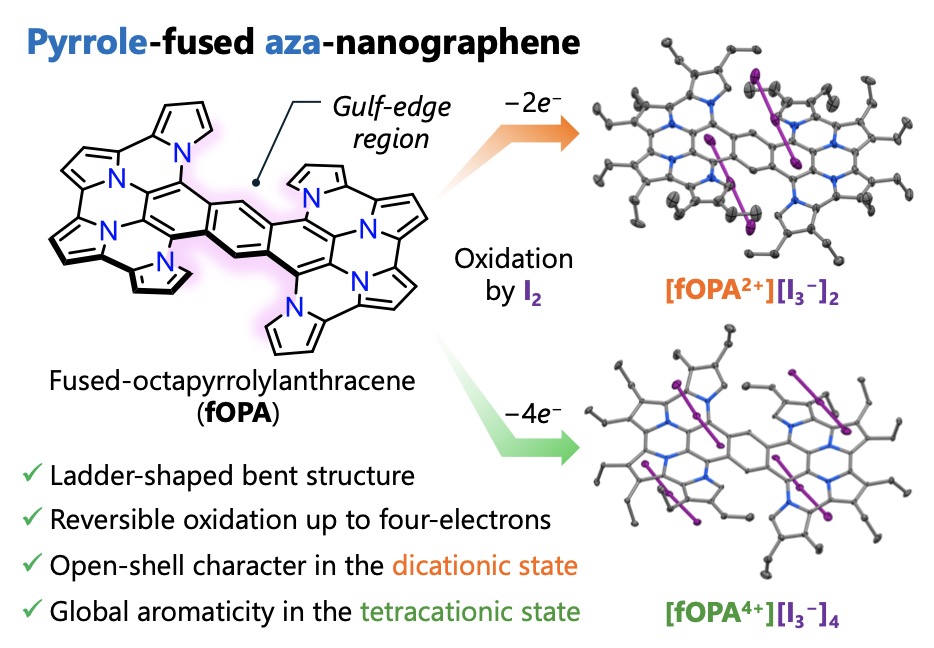
Reversible Structural and Electronic Changes of a Pyrrole-Fused Aza-Nanographene(fOPA)upon Oxidation
A pyrrole-fused aza-nanographene(fOPA)adopts a curved ladder-shaped structure and reversibly transforms from an open-shell diradical to a globally aromatic closed-shell state upon oxidation.
credit : American Chemical Society
Usage Restriction : Please get copyright permission -

A Bending Molecule that Switches Its Nature upon Oxidation
The cover features a novel aza-nanographene molecule with extensive conjugation, highlighting two “gulf-edge” sites located at specific positions. Upon two-electron oxidation, a diradical species is formed, with the distribution of unpaired electrons visually represented. The design also subtly incorporates the silhouette of Shikoku, symbolizing both the molecular architecture and the geographical origin of this research.
credit : American Chemical Society
Usage Restriction : Please get copyright permission
Contact Person
Name : Masayoshi Takase
Phone : +81-89-927-9610
E-mail : takase.masayoshi.ry@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering, Ehime University
