Toward the international unification of drug-drug interaction information
We conducted research to integrate international drug-drug interaction (DDI) information by mapping Japanese and international drug terminology. In this study, we developed a method of mapping the YJ code to RxNorm and compared the Japanese and international DDI information. The results indicated that the DDI information needs to be adjusted to be internationally consistent. It is expected that this study will facilitate the sharing and analysis of healthcare-related global data including DDI information.
The drug-drug interaction (DDI) is a phenomenon in which the efficacy of a drug is weakened or enhanced when multiple drugs are combined, the DDI can cause serious health risks to patients. In the clinical setting we are particularly careful when administering drugs with DDI relationships, but Japanese DDI information alone runs the risk of overlooking serious DDIs. Consequently, we believe that the DDI information of each country will be integrated to improve coverage. However, it has been difficult to share and analyze DDI data internationally because each country and region has different codes for identifying drugs. Thus, we aimed to map the Japanese and international drug codes to enable international data sharing.
We established a method to map the YJ code of the Japanese drug code to the RxNorm of the international standard drug terminology (Figure1). This approach has made Japanese drug information readily available to researchers and medical institutions in other countries, forming the basis for facilitating international data sharing. Furthermore, through comparison of the Japanese and international DDI information (Figure2), it became clear that agreement between the DDI severity classifications was uniformly low, indicating that clinically essential data should be added to each DDI information (Figure3). This means there are no clear internationally agreed-upon criteria for setting DDI severity, and we noted that severity information for DDI needs to be coordinated to be consistent.
The results of this study are expected to contribute greatly to the improvement of medical safety and the international integration and standardization of drug information, and to play an important role in refining DDI risk assessment and ensuring consistency of international medical data in the future.
Reference URL: https://academic.oup.com/jamia/article/31/7/1561/7676021
Bibliographic Information
Toward a unified understanding of drug-drug interactions: mapping Japanese drug codes to RxNorm concepts,
Kawakami Y, Matsuda T, Hidaka N, Tanaka M, Kimura E,
Journal of the American Medical Informatics Association, 2024, 31(7), 1561–1568,
DOI: 10.1093/jamia/ocae094, 2024 (May 17).
Media
-
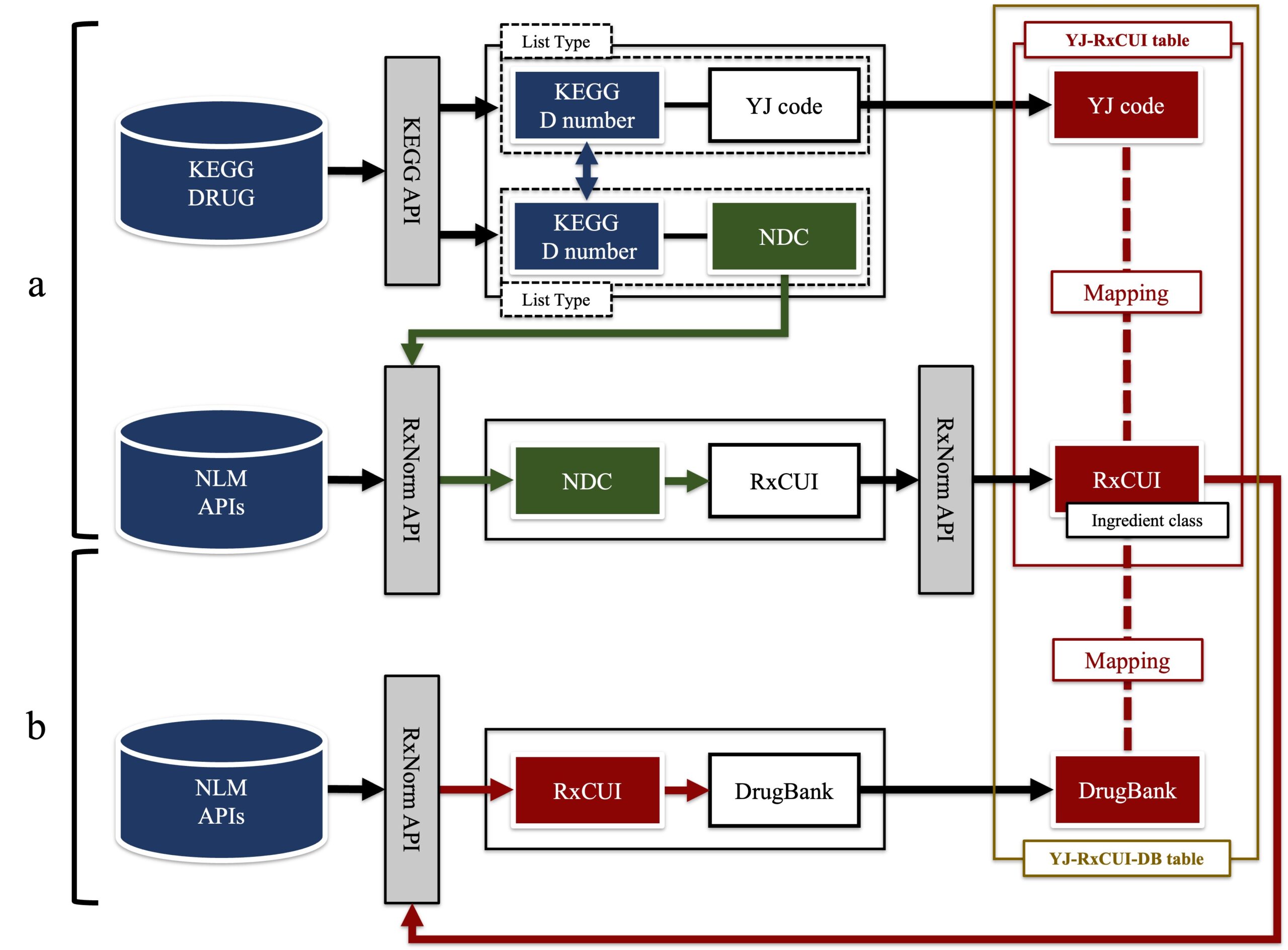
【Figure1】Method of mapping Japanese and international drug codes
Schematic diagram of the method of mapping Japanese and international drug codes. (a) To extract the correspondence table between the YJ code of the Japanese drug code and the National Drug Code (NDC) of the U.S. drug code. Next, the YJ code and RxNorm are mapped by obtaining the RxNorm related code (RxNorm Concept Unique Identifiers; RxCUI) corresponding to the NDC. (b) This shows the method of obtaining and associating other drug codes corresponding to the RxCUI.
credit : Yukinobu Kawakami, Eizen Kimura
Usage Restriction : Permission should be obtained for use. -
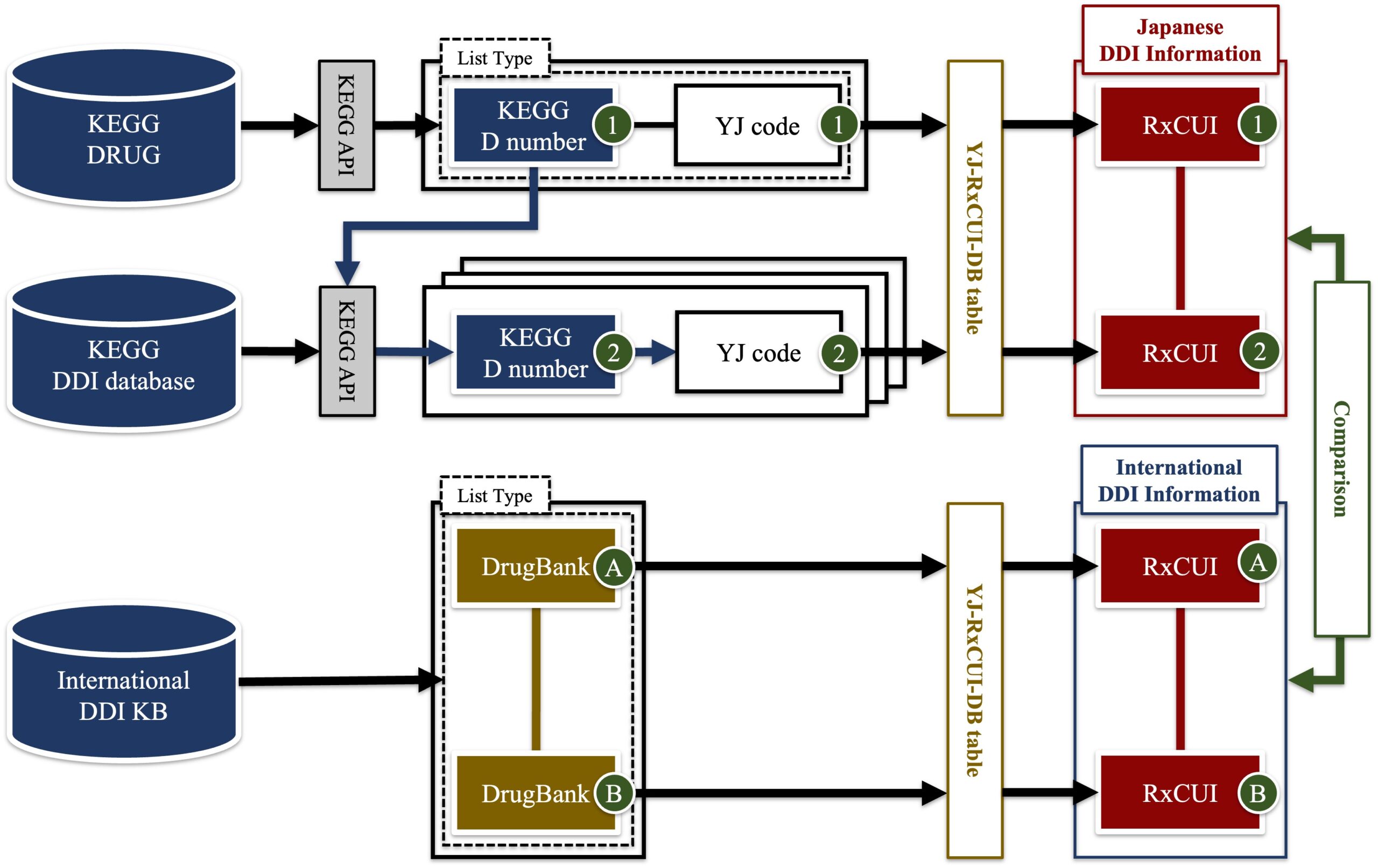
【Figure2】Method of comparing the Japanese and international DDI information
Schematic diagram of the method of comparing the Japanese and international DDI information. In the upper part of the figure, we extract the Japanese DDI information and convert the YJ code to the RxNorm related code (RxNorm Concept Unique Identifiers; RxCUI). In the lower part of the figure, we extract the international DDI information and similarly convert the drug code to RxCUI. This shows the method of comparing the Japanese and international DDI information by mapping all drugs to RxNorm.
credit : Yukinobu Kawakami, Eizen Kimura
Usage Restriction : Permission should be obtained for use. -

【Figure3】The extent of agreement among the Japanese and international DDI severity classifications
Heatmap diagram showing the extent of agreement among the DDI severity classifications. Vertical and horizontal axes are categorized as the highest class (HC), other class (OC), and no information (NI) for the severity classification of each source. The values in the color bar represent the extent of agreement by Cohen’s kappa. 0.40-0.59 indicates weak agreement; 0.21-0.39 indicates minimal agreement; and 0.00-0.20 and below 0.00 indicate no agreement.
credit : Yukinobu Kawakami, Eizen Kimura
Usage Restriction : Permission should be obtained for use.
Contact Person
Name : Yukinobu Kawakami
Phone : +81-89-960-5695
E-mail : kawakami.yukinobu.jx@ehime-u.ac.jp
Affiliation : Division of Pharmacy, Ehime University Hospital
