How Male Hormones Regulate Skeletal Muscle Function
The Androgen Receptor in Mesenchymal Progenitor Cells Drives Skeletal Muscle Mass Regulation
Androgens play a key role in regulating skeletal muscle growth and function via the androgen receptor (AR), though the precise mechanisms remain unclear. Our research reveals that AR, expressed in mesenchymal progenitors crucial for muscle homeostasis, controls muscle mass by regulating insulin-like growth factor 1 (Igf1), a major driver of muscle mass. This study highlights AR's critical role in androgen-mediated muscle growth, providing insights into potential therapeutic strategies for treating muscle atrophy such as sarcopenia.
Male hormones (androgens), as their name implies, have an important role in promoting the formation of male sexual characteristics (secondary sexual characteristics). In addition, androgens have anabolic effects, as indicated by its alias, anabolic steroid, and administration of androgens is known to cause hypertrophy of skeletal muscle. Furthermore, androgens regulate gene expression in tissues throughout the body, including skeletal muscle, by binding to a receptor called the androgen receptor (AR). However, it has remained unclear which cells in skeletal muscle are targeted by androgen and by what mechanism of action it regulates skeletal muscle mass. In this study, we focused on mesenchymal progenitors, which are important for skeletal muscle maintenance, to explore how androgens/AR regulate skeletal muscle mass.
The research group first discovered that AR was expressed in mesenchymal progenitors of skeletal muscle by immunofluorescence staining (Figure1). They then generated mice that lack AR specifically in mesenchymal progenitors (mutant mice) and observed their skeletal muscles. As a result, the mutant mice showed reduced body weight and reduced skeletal muscle weight in the hindlimbs compared to control mice (Figure1). Furthermore, when the weight of perineal skeletal muscle, known to be sensitive to androgens, was measured, the weight was significantly reduced in 14-week-old, 6-month-old, and 28-month-old mice (Figure1).
To further explore the cause of this reduction, mesenchymal progenitors were harvested from the perineal skeletal muscle of control and mutant mice and RNA sequencing was performed. The results showed that expression of insulin-like growth factor (Igf1), a protein that regulates skeletal muscle mass, was decreased in the mutant mice (Figure2). To further explore the cause of this change in gene expression, mesenchymal progenitors were harvested from hindlimb skeletal muscle of control and mutant mice and CUT&RUN was performed. The results revealed that AR binds to androgen-responsive sequences (ARE) upstream of the Igf1 gene and regulates its expression (Figure2). Finally, to confirm whether the reduction of IGF1 due to AR deficiency in mesenchymal progenitors is directly responsible for the reduction of perineal skeletal muscle mass, IGF1 was injected into the perineal skeletal muscle of mutant mice. As a result, the perineal skeletal muscle mass of mutant mice injected with IGF1 was not reduced compared to that of mutant mice injected with saline (Figure2).
These results indicate that androgens regulate skeletal muscle mass by regulating IGF1 expression via AR expressed in mesenchymal progenitors of skeletal muscle (Figure3). This finding suggests that an appropriate combination of androgens and IGF1 may lead to the development of a new treatment for sarcopenia, an age-related loss of skeletal muscle weight.
Bibliographic Information
The androgen receptor in mesenchymal progenitors regulates skeletal muscle mass via Igf1 expression in male mice,
Hiroshi Sakai, Hideaki Uno, Harumi Yamakawa, Kaori Tanaka, Ikedo, Akiyoshi Uezumi, Yasuyuki Ohkawa and Yuuki Imai,
Proceedings of the National Academy of Sciences of the United States of America, 121 (39) e2407768121,
doi:10.1073/pnas.2407768121, 2024 (September 18).
Fundings
- Japan Society for the Promotion of Science(JSPS) KAKENHI Grant Number JP21K17568, JP22H03203
- Takeda Science Foundation
- The HIRAKU-Global Program, which is funded by MEXT’s “Strategic Professional Development Program for Young Researchers”
- The MEXT Cooperative Research Project Program, Medical Research Center Initiative for High Depth Omics, and Coalition of Universities for Research Excellence Program (CURE): JPMXP1323015486 for Medical Institute of Bioregulation (MIB), Kyushu University
Media
-
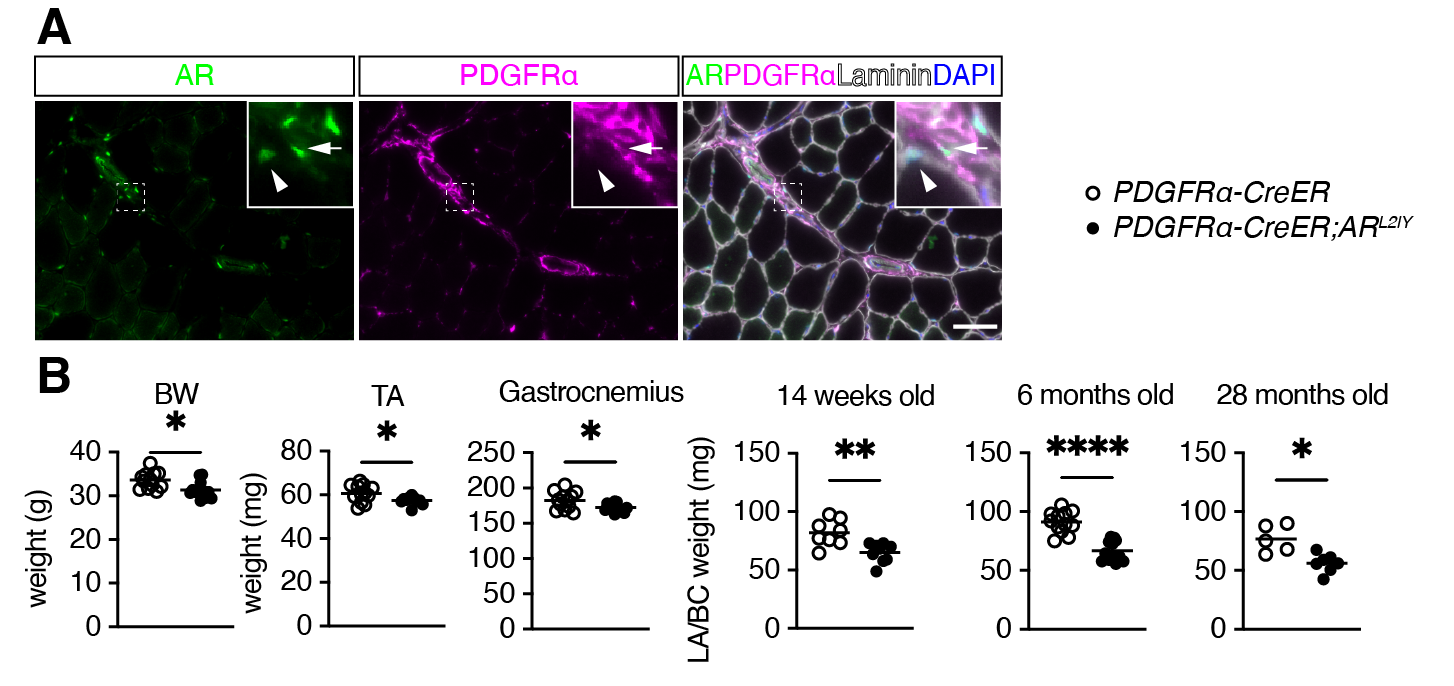
【Figure1】AR-deficient mice in mesenchymal progenitors
A. Fluorescent immunostaining of PDGFRα, showing mesenchymal progenitors, laminin surrounding skeletal muscle, and AR. Arrows indicate mesenchymal progenitors expressing AR, arrowheads indicate skeletal muscle cells expressing AR. B. Body weight (BW) and the weight of hindlimb skeletal muscle including tibialis anterior (TA) and gastrocnemius muscles at 14 weeks and perineal skeletal muscles weight in 14-week-old, 6-month-old, and 28-month-old mice.
credit : Hiroshi Sakai, Yuuki Imai, Ehime University
Usage Restriction : Permission should be obtained. -
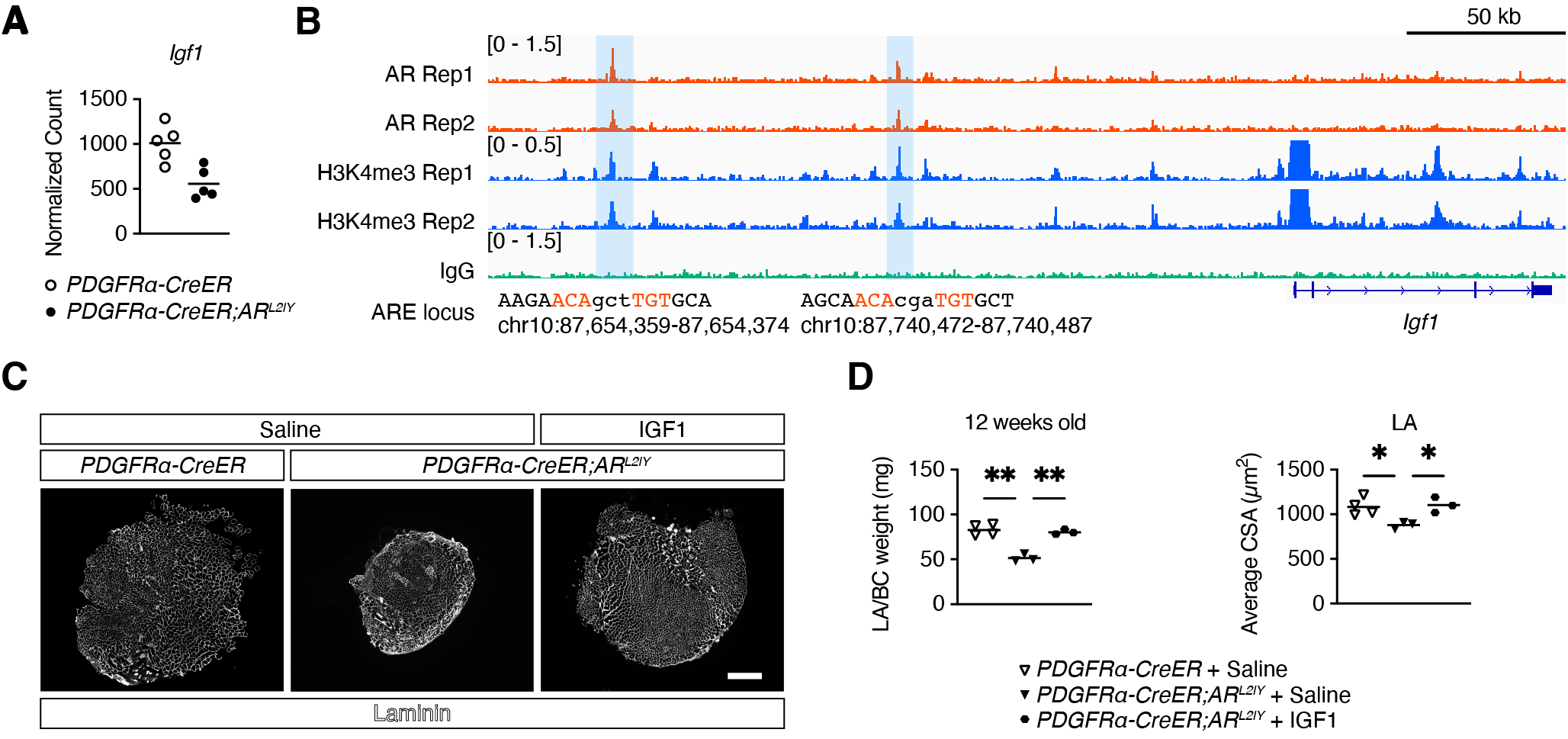
【Figure2】Regulation of IGF1 by AR in mesenchymal progenitors
A. Expression levels of Igf1 obtained by RNA sequencing. B. Location of AR bound to AREs upstream of the Igf1 gene as detected by CUT&RUN. C. Laminin fluorescence immunostaining of IGF1-injected perineal skeletal muscle. D. Weight (left) and cross-sectional area (right) of IGF1-injected perineal skeletal muscle.
credit : Hiroshi Sakai, Yuuki Imai, Ehime University
Usage Restriction : Permission should be obtained. -
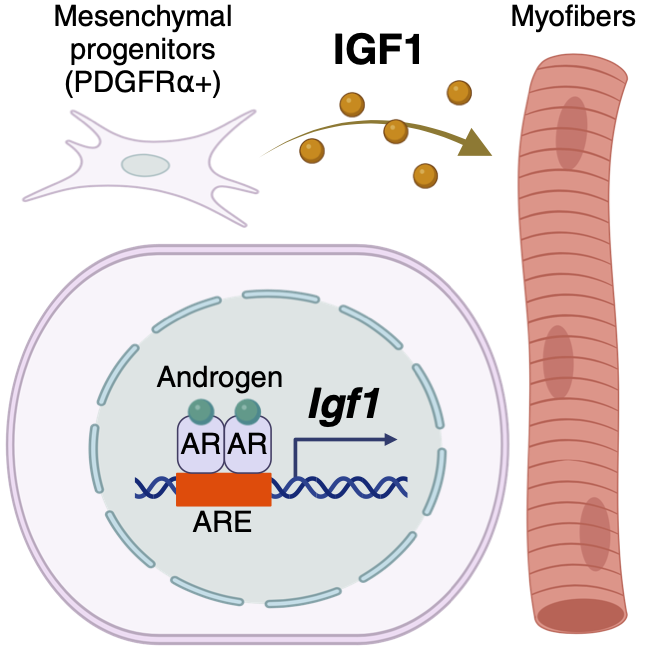
【Figure3】Conceptual diagram of skeletal muscle control mechanism by AR
The expression of AR in mesenchymal progenitors and the regulation of Igf1 expression by AR, as well as the regulation of skeletal muscle mass by IGF1, as revealed in this study.
credit : Hiroshi Sakai, Yuuki Imai, Ehime University
Usage Restriction : Permission should be obtained.
Contact Person
Name : Hiroshi Sakai
Phone : +81-89-960-5925
E-mail : sakai.hiroshi.wh@ehime-u.ac.jp
Affiliation : Division of Integrative Pathophysiology, Proteo-Science Center, Ehime University

