The first drug for severe fever with thrombocytopenia syndrome
Mortality of patients with SFTS improved by favipiravir
SFTS is a viral disease with a mortality of 27~31%. There has been no effective drug for this disease. We performed a clinical study to verify the efficacy and safety of favipiravir for SFTS patients. The results showed that the mortality of SFTS is improved by treatment with favipiravir. A corporate trial also revealed the efficacy of favipiravir for SFTS. Based on these results, favipiravir was approved for SFTS for the first time in the world in June 2024.
Severe fever with thrombocytopenia syndrome (SFTS) is a tick-borne virus infection caused by Dabie bandavirus (formerly SFTS virus, SFTSV) with high mortality. SFTS is endemic to East and Southeast Asia. The case fatality rate of patients with SFTS ranges from 27% to 31% in Japanese epidemiological surveys.
Previous research revealed that favipiravir, an antiviral drug with an inhibitory activity on the RNA-dependent RNA polymerase, inhibited replication of SFTSV in vitro and improved the mortality of SFTSV-infected mice in vivo. Based on these results, we conducted a multicenter non-randomized, uncontrolled, single-arm trial to collect data on the safety and efficacy of favipiravir in the treatment of patients with SFTS. Twenty-six patients were enrolled, of whom 23 were analyzed. The primary endpoint was 28-day mortality. Four of these 23 patients died of multi-organ failure within one week (28-day mortality rate: 17.3%). Survival probability in relation to viral load at the start of favipiravir administration was statistically significant with a virus genome load of 1 × 105 copies/mL (p = 0.01). Oral favipiravir was well tolerated in the surviving patients. The results showed that favipiravir treatment improved the case fatality rate of patients with SFTS by approximately 10% in comparison with those reported previously. Following this study, a corporate trial was conducted by FUJIFILM Toyama Chemical Co., Ltd. to re-confirm the safety and efficacy of favipiravir. The mortality rate of this trial appeared to be 13%. Analysis of this trial showed that favipiravir reduced mortality of SFTS patients by about 50%. Based on these results, favipiravir was approved in Japan as an antiviral for SFTS for the first time in the world in June 2024. Our research has made a great contribution to the treatment of SFTS.
Bibliographic Information
A multicenter non-randomized, uncontrolled single arm trial for evaluation of the efficacy and the safety of the treatment with favipiravir for patients with severe fever with thrombocytopenia syndrome.
Suemori K, Saijo M, Yamanaka A, Himeji D, Kawamura M, Haku T, Hidaka M, Kamikokuryo C, Kakihana Y, Azuma T, Takenaka K, Takahashi T, Furumoto A, Ishimaru T, Ishida M, Kaneko M, Kadowaki N, Ikeda K, Sakabe S, Taniguchi T, Ohge H, Kurosu T, Yoshikawa T, Shimojima M, Yasukawa M,
PLoS Negl Trop Dis, 22;15(2):e00091032021,
doi: 10.1371/journal.pntd.0009103, 2021(Feburary 22).
Fundings
- Japan Agency for Medical Research and Development (AMED) Research Program on Emerging and Re-emerging Infectious Diseases grant numbers 16fk0108102, 17fk0108202, and 18fk0108102
- Japan Society for the Promotion of Science(JSPS) KAKENHI Grant Number 16K09935 and 19K08931
Media
-
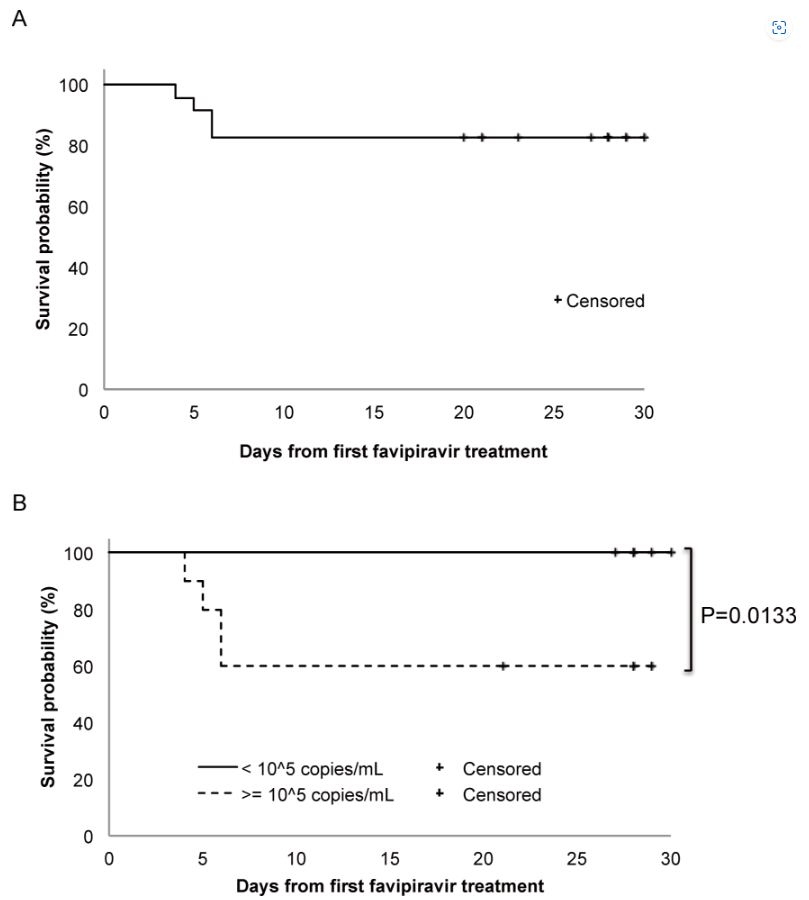
Survival rate in the 23 SFTS patients treated with favipiravir.
Four patients died within six days due to multiple organ failure, with an overall survival rate of 82.6% (A). Survival probabilities of patients with higher SFTSV viremia level (≥ 1 × 105 copies/mL, dotted line, n = 10) and that of patients with lower SFTSV viremia level (< 1 × 105 copies/mL, solid line, n = 13) are shown with a significant difference (B). This finding suggests that favipiravir should be started as early as possible to be effective.
credit : This research was originally published in PLoS Negl Trop Dis. Suemori K, et al. A multicenter non-randomized, uncontrolled single arm trial for evaluation of the efficacy and the safety of the treatment with favipiravir for patients with severe fever with thrombocytopenia syndrome. PLoS Negl Trop Dis.2021.
Usage Restriction : Please get copyright permission
Contact Person
Name : Koichiro Suemori
Phone : +81-89-960-5296
E-mail : suemori.koichiro.ix@ehime-u.ac.jp
Affiliation : Graduate School of Medicine, Ehime University
