More ancestral enzyme
Molecular evolution of enzyme beyond recruit hypothesis
・First determination of crystal structures of aconitase X by X-ray crystallographic analysis
・Evidence of a common ancestor of aconitase superfamily, appearing before the previously proposed one
・Evolutional insight of requirement of complicated metabolic pathways in primordial cell
The aconitase superfamily currently contains four functional enzymes including the archetypical aconitase (referred to as “other aconitase enzymes”), and one hypothetical aconitase X (AcnX). The aconitase enzymes catalyze the homologous stereospecific isomerization, and their three-dimensional structures and catalytic mechanisms including the [4Fe-4S] iron-sulfur cluster are very similar each other (Fig. 1a). Therefore, the aconitase superfamily (enzymes) is a typical example that is suitable for the so-called “recruitment hypothesis of enzyme evolution”; the gene duplication of multi-specific enzymes, followed by the narrowing of substrate specificity (ref. 1).
AcnX (subfamily) is further classified into “AcnXType-I” consisting of a single polypeptide, and “AcnXType-II” consisting of (fragmented) small and large polypeptide chains. In 2016, we first revealed that AcnXType-I enzyme from bacteria functions as a cis-3-hydroxy-L-proline (C3LHyp) dehydratase (Fig. 1b) (ref. 2). Furthermore, in 2018, other researchers reported that AcnXType-II enzyme from archaea functions as a mevalonate 5-phosphate (MVA5P) dehydratase (ref. 3). To elucidate their catalytic mechanisms, we herein report for the first time the crystal structures of AcnXType-I from Agrobacterium tumefaciens, a plant pathogenic bacterium (AtAcnX), and AcnXType-II from Thermococcus kodakarensis, a hyperthermophilic archaeon (TkAcnX).
AtAcnX and TkAcnX commonly consisted of four domains (fragments), and their structural frameworks of each domain were similar to their counterparts of other aconitase enzymes (Fig. 2). TkAcnX had a cuboidal [3Fe-4S] cluster, which must be derived from the [4Fe-4S] cluster unit via the loss of one iron atom, similar to other aconitase enzymes (Fig. 3a, b). Surprisingly, AtAcnX had a planar [2Fe-2S] cluster (Fig. 3c, d). Most interesting question was whether AtAcnX and TkAcnX can recognize substrates without structural similarity. Collectively, the (superimposed) backbones of C3LHyp and MVA5P were recognized by homologous residues between AtAcnX and TkAcnX (Fig. 4a), whereas their specific structural moieties by different residues (Fig. 4b, c). Since the former residues are completely conserved in other aconitase enzymes, they must be “most ancestral” active sites for aconitase superfamily. Furthermore, the acyclic MVA5P is structurally similar to those of aconitase enzymes, whereas TkAcnX recognized the substrate through homologous manners to AtAcnX, suggesting that substrate specificities (and [4Fe-4S] clusters) for TkAcnX and other aconitase enzymes had acquired each other independently.
The common ancestor of aconitase superfamily (open circle in Fig. 5), appearing before the previously proposed one (closed circle), had a similar structural framework and a few residues as active site (described above), whereas there was no [Fe-S] cluster. These results provide novel insights into the evolutionary scenario of the aconitase superfamily based on the recruitment hypothesis, and requirement of complicated metabolic pathways in primordial cell.
References
1. Jensen, R. A. (1976) Enzyme recruitment in evolution of new function. Annu. Rev. Microbiol. 30, 409-425.
2. Watanabe, S., Tajima, K., Fujii, S., Fukumori, F., Hara, R., Fukuda, R., Miyazaki, M., Kino, K., Watanabe, Y. (2016) Functional characterization of aconitase X as a cis-3-hydroxy-L-proline dehydratase. Sci. Rep. 6, 38720.
3. Hayakawa, H., Motoyama, K., Sobue, F., Ito, T., Kawaide, H., Yoshimura, T., Hemmi, H. (2018) Modified mevalonate pathway of the archaeon Aeropyrum pernix proceeds via trans-anhydromevalonate 5-phosphate. Proc. Natl. Acad. Sci. U S A. 115, 10034-10039.
Reference URL: https://www.nature.com/articles/s42003-021-02147-5
Bibliographic Information
Crystal structures of aconitase X enzymes from bacteria and archaea provide insights into the molecular evolution of the aconitase superfamily, Seiya Watanabe, Yohsuke Murase, Yasunori Watanabe, Yasuhiro Sakurai, Kunihiko Tajima, communications biology, 4, 687, doi.org/10.1038/s42003-021-02147-5, 2021 (June 7).
Media
-
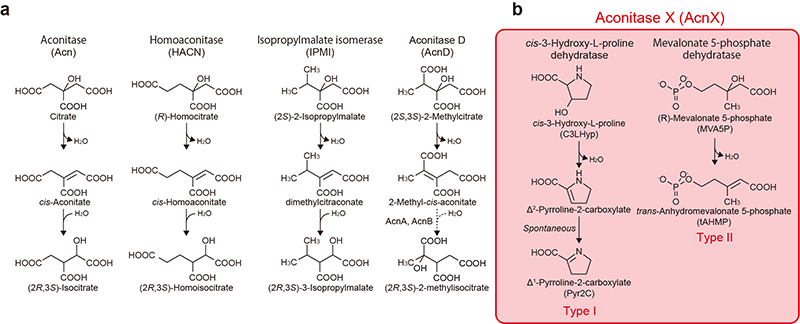
Figure 1. Schematic reactions of aconitase superfamily members.
(a) Four known functional enzymes including the archetypical aconitase. (b) Two recently functionally characterized AcnX enzymes.
credit : Seiya Watanabe, Ehime University
Usage Restriction : Please get copyright permission -
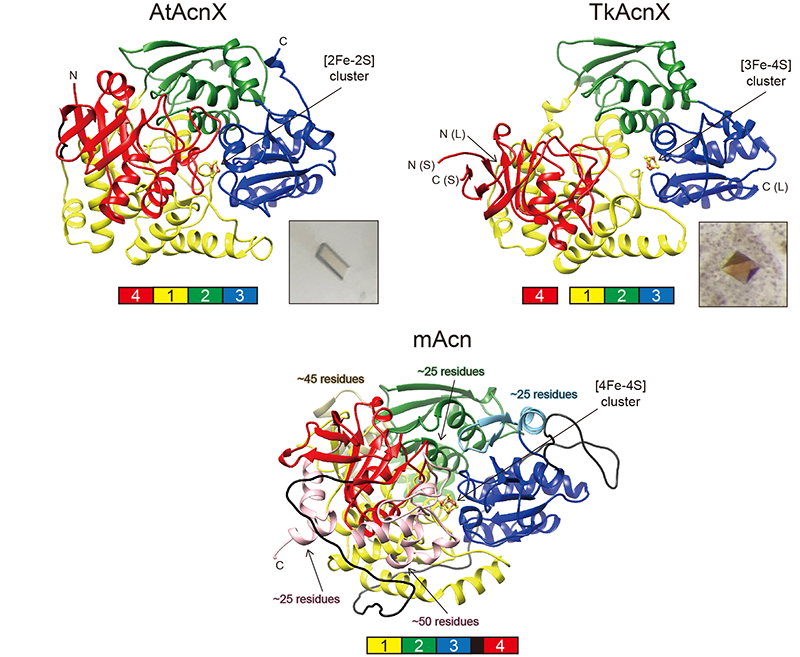
Figure 2. Overall crystal structures of AtAcnX, TkAcnX, and mAcn (aconitase from bovine mitochondria).
These molecules commonly contain the indicated [Fe-S] cluster, and consist of four distinct domains 1 (yellow), 2 (green), 3 (blue), and 4 (red) and a linker region (black). The small subunit of TkAcnX corresponds to fragmented domain 4. In comparisons with AcnX, several large insertions in domains 1-4 of mAcn were colored in ocher, light-green, light-blue, and pink, respectively.
credit : Seiya Watanabe, Ehime University
Usage Restriction : Please get copyright permission -
![Figure 3. Analysis of the [Fe-S] cluster in AcnX.](https://research.ehime-u.ac.jp/mng_79156/wp-content/uploads/2021/07/files-62353.png)
Figure 3. Analysis of the [Fe-S] cluster in AcnX.
The observed [3Fe-4S] cluster of TkAcnX in the apo-form (a) and in complex with MVA5P (b). The observed [2Fe-2S] cluster of AtAcnX in the apo-form (c) and in complex with C3LHyp (d).
credit : Seiya Watanabe, Ehime University
Usage Restriction : Please get copyright permission
Contact Person
Name : Seiya Watanabe
Phone : +81 89-946-9848
E-mail : irab@agr.ehime-u.ac.jp
Affiliation : Graduate School of Agriculture
