The Formation of Switchable and Metastable Discrete Structures through Chiral Self-sorting
The Formation of Switchable and Metastable Discrete Structures through Chiral Self-sorting
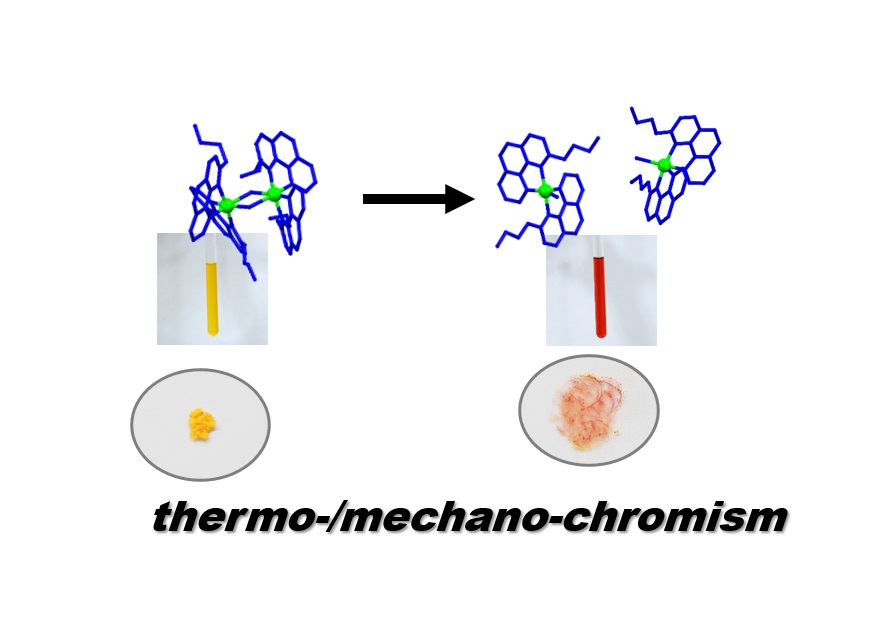
This paper describes chiral coordination dimers that emerge based on chiral self-sorting andexhibit reversible thermo-/mechano-chromism originating from monomer-dimer transformation. The homochiral dimer is comprised of a coordinatively unsaturated iridium(III) complex. This work illustrates the potential of chiral self-sorting as an important parameter in the design of dynamic molecular systems for various applications in discrete supramolecular chemistry, and is of special interest to those engaged in research on stereochemistry, molecular dynamics, functional nanomaterials and catalysts.
This paper describes chiral coordination dimers that emerge based on effectively exclusive chiral self-sorting. The complex also exhibits thermo-/mechano-chromism originating from monomer-dimer transformation. The homochiral dimer is comprised of a coordinatively unsaturated iridium(III) complex, which features an n-butyl-substituted benzo[h]quinoline moiety and helical chirality at the metal center. Construction of the appropriate binding model and analysis of the fundamental physical parameters based on spectroscopic data reveal that the strong preference for homochiral dimerization is an entropic-driven effect originating from steric repulsions of alkyl chains in the coordination sphere of the corresponding heterochiral dimer. Furthermore, the metastable nature of dimer crystals allows for color variation (from yellow to red) upon mechanical cleavage of its coordination bonds (i.e., dimer-to-monomer transformation). This feature might be exploited for the dynamic control of coordination geometry and related functionalities, such as catalytic applications. Emergence of strong homochiral self-sorting preference and connected thermo-/mechano-chromic behaviour is based on matched propeller-shaped chirality and subtle steric repulsions of substituents that render particular homochiral dimers switchable and metastable.
This work provides substantial insight into chiral self-sorting in discrete supramolecular systems and its application in the rational design of switchable and metastable dynamic molecular structures with potential as advanced catalysts, sensors, or optoelectronic devices.
Reference URL: https://doi.org/10.1021/jacs.3c05866
Bibliographic Information
Thermo-/mechano-chromic Chiral Coordination Dimer: Formation of Switchable and Metastable Discrete Structure through Chiral Self-sorting, Kazuyoshi Takimoto,* Takumi Shimada, Kazuhiko Nagura, Jonathan P. Hill, Takashi Nakanishi, Hidetaka Yuge, Shinsuke Ishihara,* Jan Labuta,* Hisako Sato*, Journal of the American Chemical Society, Accepted, 2023.
Fundings
- JSPS KAKENHI Grants JP17H03044, JP20K21090, JP22H02033, JP19K05229, JP23K13766 Grant-Aid for JSPS Fellows JP21J15602
- Kitasato University Research Grant for Young Researchers
Media
Contact Person
Name : Hisako Sato
Phone : +81-89-927-9599
E-mail : sato.hisako.yq@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering, Ehime University, Former Professor (Currently, Research Fellow (Project Leader), Faculty of Science)