Development of atactic C-C main chain polymer with a high melting point
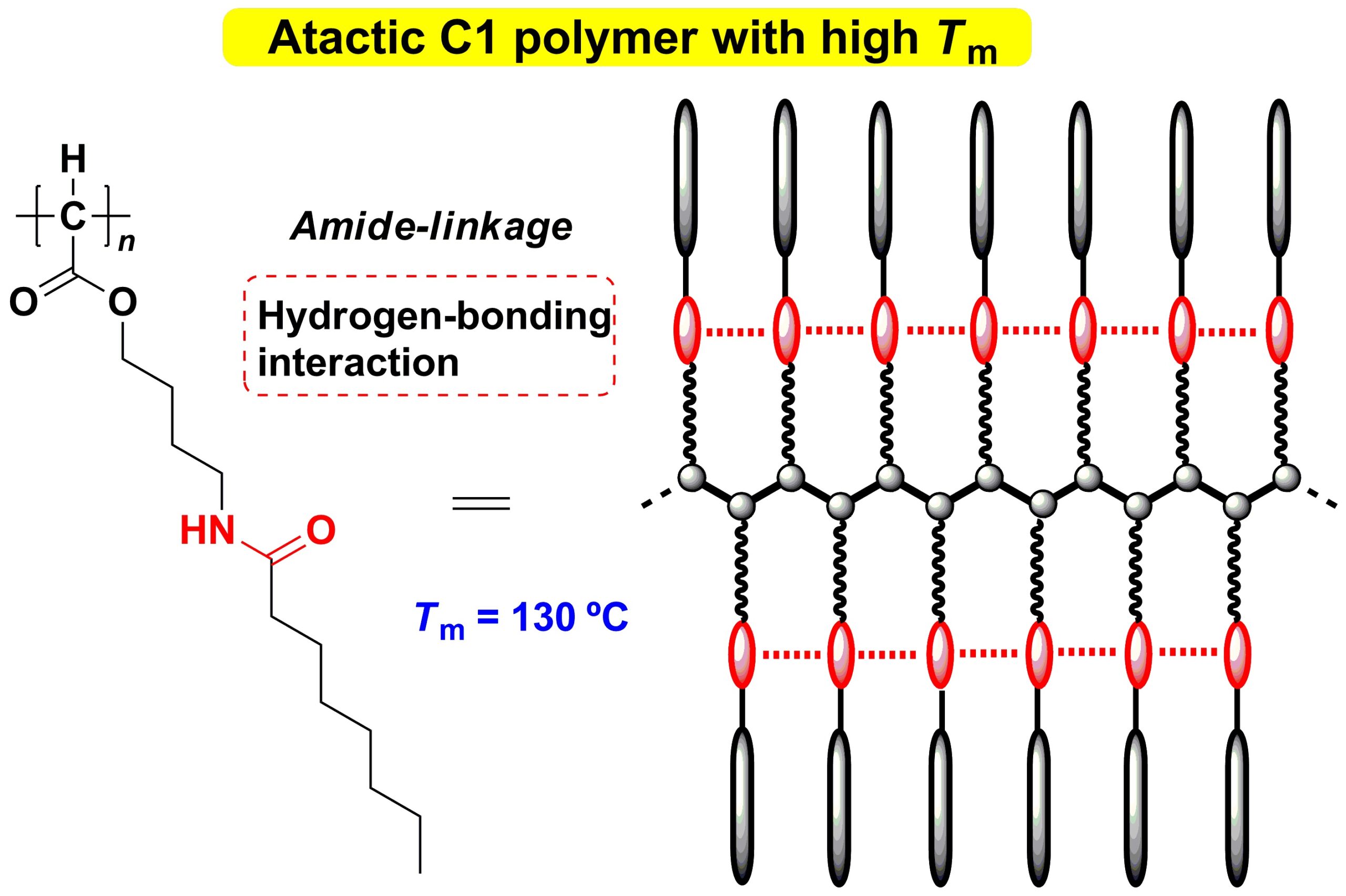
The Ehime University research team directed by H. Shimomoto and E. Ihara succeeded in preparing atactic C1 polymers with a high melting point of up to 130 ℃ by utilizing a hydrogen-bonding interaction among amide-linkages incorporated into the polymer side-chains. The C1 polymer, in which all the main chain carbon atoms have an alkoxycarbony group, was prepared by a method originated by our research team. This achievement will contribute to the development of new functional polymer materials.
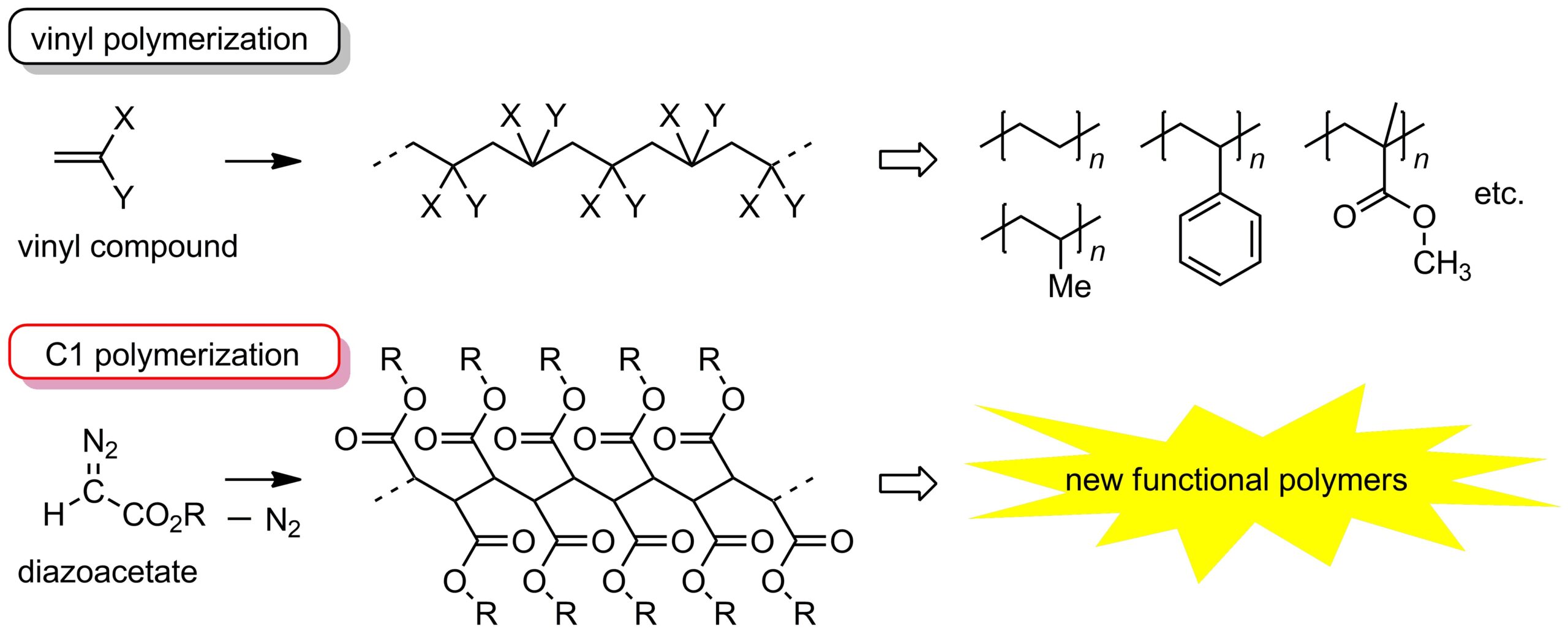
Polymers with carbon–carbon (C-C) bonds (C-C polymers) in the main chain skeleton such as polyethylene and polypropylene are important industrial materials and utilized as common plastics. Most C-C polymers have been prepared by vinyl polymerization, where starting materials (monomers) with a C=C double bond such as ethylene and propylene were used for their production. On the other hand, we have succeeded in developing an alternative method for preparing C-C polymers using diazoacetate as a monomer, where the C-C bonds are constructed from C1 units derived from the monomer. The polymers obtained by the latter method (C1 polymers) are expected to become new polymer materials with useful properties that cannot be obtained by vinyl polymerization.
In general, imparting high crystallinity to polymers is an effective method for improving the strength and durability of the materials, and high crystallinity is achieved by high stereoregularity in the polymer main chain. Accordingly, polymers with low stereoregularity (atactic polymers) tend to be less valuable materials; for example, polypropylene can be a useful material only when its C-C bonds are highly stereoregulated. As for C1 polymers, whereas highly stereoregulated polymers with high crystallinity have been reported, atactic C1 polymers are amorphous with a relatively low melting point, as well.
In this study, we have succeeded in synthesizing an atactic C1 polymer with a high melting point by incorporating an amide-linkage into its side-chain. As we expected, the amide-linkages suppress the side-chain mobility because of an inter- and intrachain hydrogen-bonding interaction among them, resulting in raising the melting point up to 130 ℃. On the other hand, the incorporation of the identical amide-linkage into side-chains of a vinyl polymer was not effective in raising its melting point; thus, the higher melting point of the C1 polymer was realized because the dense-packing of its side chains greatly enhances the hydrogen-bonding interaction.
These results provide a new insight into the thermal properties of C-C polymers and is expected to be applied to new polymer material design. This achievement appeared in the electronic version of Macromolecules published by the American Chemical Society on June 12, 2023.
Bibliographic Information
Effect of the Alkyl Side-Chain Structure on Melting Point of Atactic Poly(alkoxycarbonylmethylene)s: Incorporation of Amide-Linkage Leading to Polymers with High Melting Point
Hiroaki Shimomoto,* Itsuki Katashima, Hirokazu Murakami, Tomomichi Itoh, and Eiji Ihara*
Macromolecules, 2023, 56, 4639–4648
DOI: 10.1021/acs.macromol.3c00635
Fundings
- Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number JP18H02021, JP19K05586, JP19K22219, JP21H01988, JP22K05219
Media
Contact Person
Name : Hiroaki Shimomoto
Phone : +81-89-927-9949
E-mail : shimomoto.hiroaki.mx@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering