A Demonstration of Substituent Effects in Anti-aromatic Compounds
●Diverse synthesis of stable anti-aromatic compounds (homoHPHAC+)
●Elucidation of global anti-aromaticity (aromaticity) in homoHPHAC+ (homoHPHAC3+) with electron-accepting to electron-donating substituents
Circularly conjugated compounds with 4n+2 pi-electrons are known as aromatic compounds. They are generally stable and are therefore found in our surroundings. On the other hand, anti-aromatic compounds with 4n pi-electrons have been conventionally considered unstable, and the creation of stable anti-aromatic compounds has been one of the challenging issues in organic chemistry. Several studies on the synthesis, isolation, and characterization of stable and clearly anti-aromatic compounds have been reported in recent years. In general, anti-aromatic compounds are considered to be more susceptible to substituents than aromatic compounds because of their narrower HOMO-LUMO gap. However, there has been no systematic study of such substituent effects in anti-aromatic compounds.
This research group has been conducting studies on the synthesis and properties of hexapyrrolohexaazacoronene (HPHAC), a nitrogen-containing polycyclic aromatic compound consisting of pyrrole. Furthermore, homoHPHAC, a pi-extended analog of HPHAC, was reported to show global anti-aromaticity as a monocation and global aromaticity as a trication. In this study, a new synthetic method for homoHPHACs using Friedel-Crafts-type intramolecular condensation reactions was developed, and a series of compounds with electron-donating to electron-accepting substituents were synthesized. The effects of substituents on structural, optical, redox, and antiaromatic (aromatic) properties were demonstrated. In conjunction with computational chemistry, it was shown that both anti-aromatic (monocation) and aromatic (tricationic) properties were the strongest in compounds with electron-accepting substituents.
Various approaches to the use of organic compounds as electronic materials are being investigated from the viewpoints of reducing environmental impact and providing versatility in functional control. The attempt to control electronic properties by introducing substituents into anti-aromatic compounds is expected to provide new design guidelines for molecular materials.
Reference URL: https://pubs.rsc.org/en/content/articlelanding/2023/sc/d2sc07037e
Bibliographic Information
Substituent Effects on Paratropicity and Diatropicity in π-Extended Hexapyrrolohexaazacoronene,
Masayoshi Takase, Toranosuke Takata, Kosuke Oki, Shigeki Mori, and Hidemitsu Uno,
Chemical Science, 2023, 14, 7036-7043.
DOI: 10.1039/D2SC07037E (June 06, 2023)
Fundings
- JSPS KAKENHI JP23H03964, JP20H02725, JP19K05422
- The Takahashi Industrial and Economic Research Foundation
- The Takahashi Industrial and Economic Research Foundation
- Ehime University, “Research Unit on Molecular Materials Science for Toroidal π-Electron Systems”
Media
-
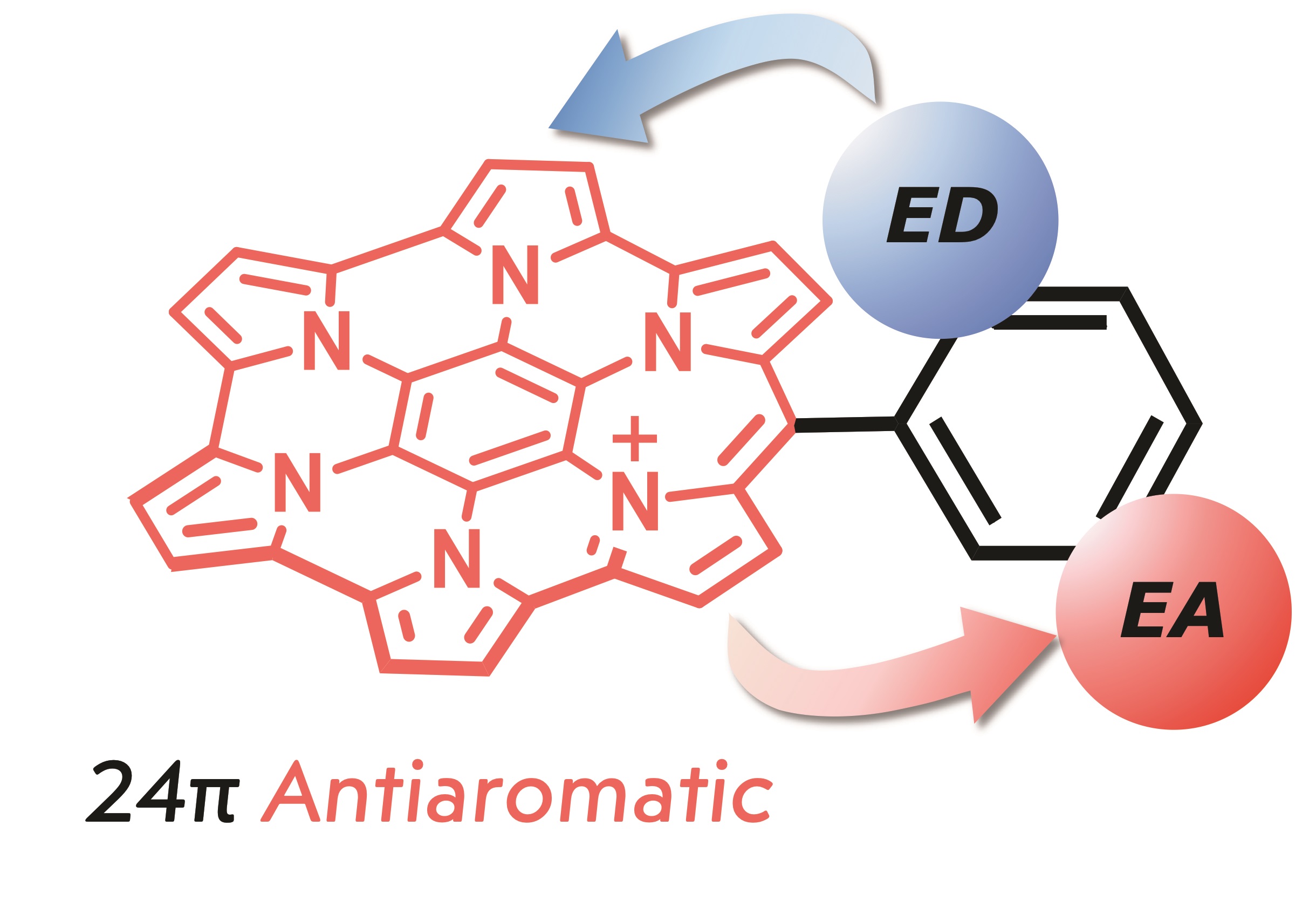
Demonstration of Substituent Effects in Anti-aromatic Compounds
π-Expanded homoHPHACs with electron-donating (ED) and electron-accepting (EA) substituents change the intensity of their paratropicity as well as optical and redox properties.
credit : Masayoshi Takase
Usage Restriction : Please get copyright permission
Contact Person
Name : Masayoshi Takase
Phone : +81-89-927-9610
E-mail : takase.masayoshi.ry@ehime-u.ac.jp
Affiliation : Graduate School of Science and Engineering
